The mass percent formula is a fundamental concept; it is used to express the relative amount or concentration of a component within a solution. It represents the mass of a solute present in a given quantity of solution, expressed as a percentage.
- The Mass percentage can define the solution components particular solution.
- The solute is the component that is concentrated on in a particular mixture.
- Many solutes together form a solution, simply termed as the solution is a mixture of solutes.
- And to determine how much mass of the solute we are referring to is present in the solution’s total mass, we use mass percent.
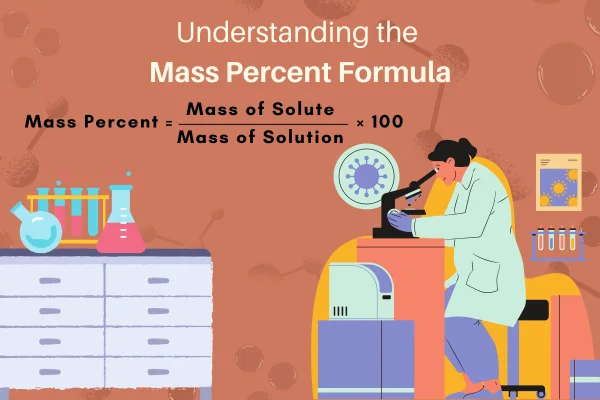
The Mass Percent Formula can be stated as:
Mass Percent = (Mass of Solute / Mass of Solution) × 100
This formula allows chemists to quantify the amount of a specific substance in a solution and determine its proportionate representation.
- This formula is used to solve the molar mass for the mass of each element present in one mole of the compound. The mass percentage is also referred to as w/w % (Percent by Weight).
- Three different formulas are based on this equation: Mass by Mass Percentage, Mass by Volume Percentage, and Volume by Volume Percentage.
Significance of the Mass Percent Formula:
The mass percent formula is particularly useful in various scientific fields, including pharmaceuticals, environmental science, and chemical analysis. It aids in determining the concentration of a particular substance, verifying the quality of solutions, and assessing the efficiency of chemical reactions. Additionally, the mass percent formula is a valuable tool in understanding solubility, as it provides insights into the amount of solute dissolved in a given solvent. By utilizing this formula, chemists can ensure accuracy in experimental procedures and perform necessary adjustments when required.
Examples of Mass Percent Calculation
Example 1: Calculating the Mass Percent of Salt in a Solution
Suppose we have 50 grams of a salt solution and find that 8 grams of salt (solute) is dissolved within it. To determine the mass percent of the salt in the solution, we can use the formula:
Mass Percent = (8 g / 50 g) × 100 = 16%.
Example 2: Determining the Mass Percent of Ethanol in a Mixture
Consider a mixture that contains 60 grams of ethanol (solute) dissolved in 240 grams of water (solvent). To calculate the mass percent of ethanol, we can apply the formula:
Mass Percent = (60 g / 300 g) × 100 = 20%.
Example 3: Estimating the Mass Percent of Carbon Dioxide in a Gas Mixture
Suppose a gaseous mixture contains 20 grams of carbon dioxide (solute) and 80 grams of oxygen (solvent). To determine the mass percent of carbon dioxide in the mixture, we can utilize the formula:
Mass Percent = (20 g / 100 g) × 100 = 20%
In all the examples, the mass percent indicates the relative concentration of the solute within the solution or mixture.
The mass percent formula is an essential tool for chemists and researchers, enabling them to determine the concentration of a specific substance within a solution. By employing this formula, the proportionate representation of the solute can be quantified accurately. Understanding the mass percent formula helps in assessing the quality of solutions, evaluating the efficiency of chemical reactions, and predicting solubility.

